Brønsted acid-catalyzed aromatic annulation of alkoxyallenes with naphthols: a reaction sequence to larger π-conjugated naphthopyrans with aggregation-induced emission characters†
Abstract
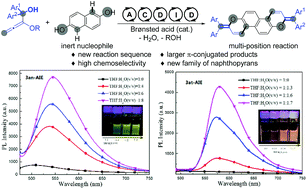
A practical and readily scalable reaction sequence was developed for the straightforward synthesis of a new family of larger π-conjugated naphthopyrans by a Brønsted acid-catalyzed aromatic annulation of alkoxyallenes with inert naphthols. The cascade pathway involves allylation/cyclization/debenzyloxylation/isomerization/dehydration. The new class of solid state diphenylmethylene substituted naphthopyrans are fluorescent emissive and proved to have aggregation-induced emission (AIE) behavior.


 Please wait while we load your content...
Please wait while we load your content...