Transforming atmospheric CO2 into alternative fuels: a metal-free approach under ambient conditions†
Abstract
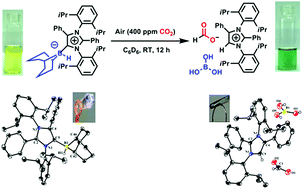
This work demonstrates the first-ever completely metal-free approach to the capture of CO2 from air followed by reduction to methoxyborane (which produces methanol on hydrolysis) or sodium formate (which produces formic acid on hydrolysis) under ambient conditions. This was accomplished using an abnormal N-heterocyclic carbene (aNHC)–borane adduct. The intermediate involved in CO2 capture (aNHC-H, HCOO, B(OH)3) was structurally characterized by single-crystal X-ray diffraction. Interestingly, the captured CO2 can be released by heating the intermediate, or by passing this compound through an ion-exchange resin. The capture of CO2 from air can even proceed in the solid state via the formation of a bicarbonate complex (aNHC-H, HCO3, B(OH)3), which was also structurally characterized. A detailed mechanism for this process is proposed based on tandem density functional theory calculations and experiments.
- This article is part of the themed collections: Celebrating the Chemical Sciences in India - Leaders in the Field Symposium 2020 and How can chemistry adapt to a low carbon future


 Please wait while we load your content...
Please wait while we load your content...