A H-bond stabilized quinone electrode material for Li–organic batteries: the strength of weak bonds†
Abstract
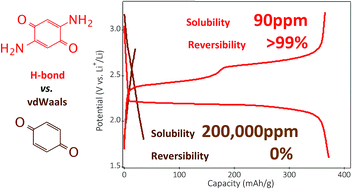
Small organic materials are generally plagued by their high solubility in battery electrolytes. Finding approaches to suppress solubilization while not penalizing gravimetric capacity remains a challenge. Here we propose the concept of a hydrogen bond stabilized organic battery framework as a viable solution. This is illustrated for 2,5-diamino-1,4-benzoquinone (DABQ), an electrically neutral and low mass organic chemical, yet with unusual thermal stability and low solubility in battery electrolytes. These properties are shown to arise from hydrogen bond molecular crystal stabilization, confirmed by a suite of techniques including X-ray diffraction and infrared spectroscopy. We also establish a quantitative correlation between the electrolyte solvent polarity, molecular structure of the electrolyte and DABQ solubility – then correlate these to the cycling stability. Notably, DABQ displays a highly reversible (above 99%) sequential 2-electron electrochemical activity in the solid phase, a process rarely observed for similar small molecular battery chemistries. Taken together, these results reveal a potential new strategy towards stable and practical organic battery chemistries through intramolecular hydrogen-bonding crystal stabilization.
- This article is part of the themed collection: Most popular 2018-2019 energy articles


 Please wait while we load your content...
Please wait while we load your content...