Selective deoxygenation of nitrate to nitrosyl using trivalent chromium and the Mashima reagent: reductive silylation†
Abstract
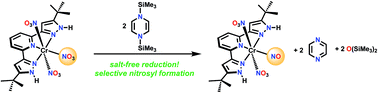
1,4-Bis(trimethylsilyl)-1,4-diaza-2,5-cyclohexadiene is an effective silyl transfer reagent towards the oxygen of nitrate coordinated to Cr(III) in a pincer complex. Two nitrate oxygens are removed to give the 17 valence electron octahedral complex (H2L)Cr(NO3)2(NO). This is shown by a variety of spectroscopic methods, together with DFT, to be a Cr(I) complex with a linear CrNO unit. This work also identifies future applications of this reductive silylation process.


 Please wait while we load your content...
Please wait while we load your content...