Influence of structure–activity relationships on through-space intervalence charge transfer in metal–organic frameworks with cofacial redox-active units†
Abstract
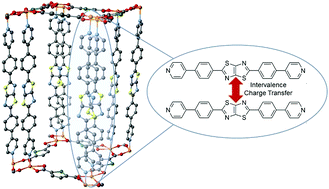
Understanding charge transfer in redox-active metal–organic frameworks (MOFs) is of fundamental importance given the potential of these materials to be used in myriad applications including porous conductors, electrocatalysts and battery materials, amongst others. An important challenge is quantifying the spectroscopic features of these materials in order to elucidate their charge transfer properties. Herein, two topologically related Zn(II) and Cd(II) frameworks, [Zn2(DPPTzTz)2(SDC)2] (1-Zn) and [Cd2(DPPTzTz)2(SDC)2] (2-Cd) (where DPPTzTz = 2,5-bis(4-(4-pyridinyl)phenyl)thiazolo[5,4-d]thiazole and SDC = selenophene-2,5-dicarboxylate), incorporating cofacially stacked pairs of redox-active DPPTzTz ligands are presented. The differences in the through-space intervalence charge transfer properties of the mixed-valence forms of the two frameworks generated upon solid state spectroelectrochemical reduction are quantified using Marcus–Hush theory. Further, charge transfer theory is applied to calculate electron mobilities in both extended framework systems. A larger electronic coupling constant, Hab, of 118 cm−1 corresponding to an electron mobility, k, of 6.02 × 108 s−1 was observed for the Zn(II) analogue compared to the Cd(II) analogue (Hab = 61.2 cm−1 and k = 2.22 × 108 s−1) and was correlated primarily with the larger cofacial stacking distance and increasingly offset stacking geometry between DPPTzTz ligands in the latter. Establishing structure–activity relationships in electroactive MOFs, in addition to methods for quantifying their charge transfer properties, represents an important advance in fine tuning solid state materials for device applications.
- This article is part of the themed collection: Most popular 2019-2020 inorganic, main group and crystal engineering chemistry articles


 Please wait while we load your content...
Please wait while we load your content...