A streamlined synthesis of the neurosteroid 3β-methoxypregnenolone assisted by a statistical experimental design and automation†
Abstract
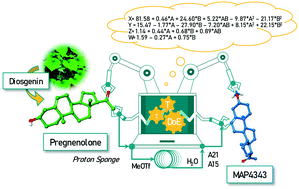
The potential of integrating flow synthesizers, statistical design of experiments and automation has been exemplified to realize the streamlined etherification of pregnenolone to the neurosteroid 3β-methoxypregnenolone (MAP4343). The use of Proton Sponge™, online reaction monitoring, work-up, product purification, and reagent recovery are additional key features of the present study. The optimized single-step process was validated by conducting a multistep sequence that has enabled the continuous flow synthesis of MAP4343 on a laboratory scale with an overall yield of 64% via six steps from the readily available plant-derived diosgenin.


 Please wait while we load your content...
Please wait while we load your content...