Impact of cation redox chemistry on continuous hydrothermal synthesis of 2D-Ni(Co/Fe) hydroxides
Abstract
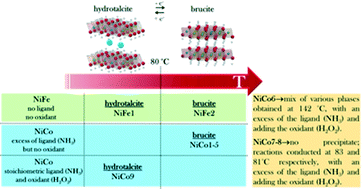
Continuous hydrothermal flow synthesis (CHFS) is a facile, upscalable and cost-efficient synthetic method enabling the nanostructuring of advanced functional materials in steady conditions, i.e. not in batch synthesis. In this paper, we use CHFS to crystallize NiCo- and NiFe-hydroxides in water solution with 2D nanofeatures. By tuning the synthetic parameters, we disclose the key role of the cation redox chemistry in the transition between two competitive phases: from 2D-nanoplatelets of brucite to layered double hydroxides (LDH). For controlling the precipitation of different Ni, Fe, Co-hydroxide phases, we propose the combined use of an oxidizing (H2O2) and a complexing (NH3) agent. At temperatures as low as 80 °C, the presence of H2O2 and a low concentration of NH3 favour the Ni2+/Co3+ over Ni2+/Co2+ oxidation states, shifting the product structure from brucite phase (temperatures > 80 °C) to LDH. Conversely, for the NiFe-hydroxides the transition from LDH (temperatures ≤ 80 °C) to brucite phase (temperatures > 80 °C) is controlled by the reaction temperature only. Due to the high stability of Fe3+, the synthesis of NiFe products by CHFS does not require oxidizing and complexing agents, resulting in a robust process for large-scale production.


 Please wait while we load your content...
Please wait while we load your content...