Nucleophilic construction of sulfate bonds: simplified access to polysulfates and polysulfonates†
Abstract
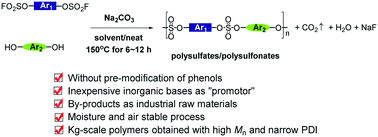
We herein report a concise process for the quantitative synthesis of polysulfates and polysulfonates through nucleophilic substitution between aryl phenols and aryl fluorosulfates in the presence of inorganic bases such as K2CO3, Na2CO3, K3PO4, Cs2CO3, etc. The reaction we developed was carried out without pre-preparation of phenolates or further modification of aryl phenols with “Si” groups (for example, TMS and TBS groups), sharply reducing the production costs both in lab research and industrial manufacture. The aryl phenolates were formed in situ, and the process was moisture and air stable. By applying this process, polysulfates and polysulfonates with the properties of high molecular weight, narrow polydispersity, kilogram scale and functional group tolerance were prepared. The byproducts of our process were water and fluorides. Several physical and chemical properties of the polymer were evaluated. Also, P-1 could be synthesized through a one-pot process in the presence of BPA, base and SO2F2. The distinct advantages of our protocol make it a more valuable mode for future industrial applications for the synthesis of polysulfates and polysulfonates, as well as the formation of sulfate bonds.
- This article is part of the themed collection: 2019 Reaction Engineering in China


 Please wait while we load your content...
Please wait while we load your content...