An efficient process for the Cu(0)-mediated synthesis and subsequent chain extension of poly(methyl acrylate) macroinitiator†
Abstract
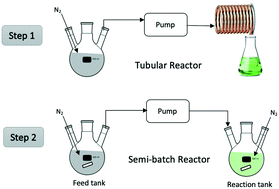
Low dispersity poly(methyl acrylate) with a target degree of polymerization of 10 (poly(MA)10) was produced in both batch and a continuous tubular reactor at room temperature using Cu(0) catalysis. The resulting polymer solution could be stored for over a week while maintaining excellent activity as a macroinitiator for the further growth of poly(acrylate) chains in both batch and semi-batch reactor systems. The chain-extensions were done without addition of further copper to the system using ascorbic acid as a reducing agent; the best performance (>90% conversion with excellent control) was achieved when a fraction of the ascorbic acid was added to the reactor at the start of reaction, with the rest fed in a semi-batch fashion along with monomer over three hours. Semi-batch chain extensions of poly(MA)10 with butyl acrylate and poly(ethylene glycol) methyl ether acrylate (PEGMEA) were conducted, and a tri-block polymer was also synthesized. The feasibility of applying this procedure to other monomers was demonstrated through the synthesis and chain extension of poly(PEGMEA)10. The combination of the tubular reactor to produce macroinitiator and subsequent semi-batch chain extension is proposed as a versatile process to efficiently produce block copolymers at an industrial scale.


 Please wait while we load your content...
Please wait while we load your content...