New process for producing butane-2,3-dione by oxidative dehydrogenation of 3-hydroxybutanone†
Abstract
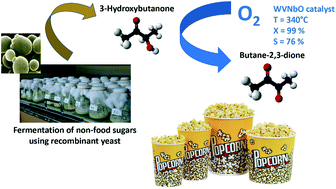
Reaction of 3-hydroxybutanone in air has been studied with and without a catalyst under atmospheric pressure and at temperatures between 523 and 673 K. The results revealed that the oxidative dehydrogenation reaction into butane-2,3-dione is thermodynamically preferred to the dehydration reaction, which did not occur, and to the oxidation into acetic acid, which occurs partially. The oxidative dehydrogenation proceeds via homogeneous radical mediated pathways in air and almost total conversion with a selectivity of about 85% can be obtained at 738 K. DFT calculations have been performed to provide insights into the homogeneous pathways. O2 and its derivatives are considered to take part in the reaction as H acceptors. The first step is the de-protonation of the secondary carbon atom, then mainly followed by the de-protonation of the hydroxyl group to finally obtain butane-2,3-dione. The cleavage of the intermediate radical leading to acetic acid is possible but not the most favorable. Tungsten oxides with bronze type structures and containing V and Nb have been studied as catalysts and turned out to be efficient to accelerate the reaction but did not lead to a significant increase in selectivity to butane-2,3-dione.


 Please wait while we load your content...
Please wait while we load your content...