Reaction pathways and kinetics for tetra-alanine in hot, compressed liquid water†
Abstract
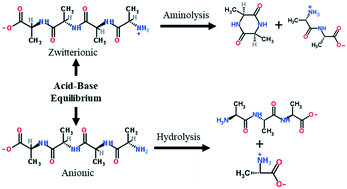
Proteins are abundant biochemical components of microalgae and food wastes that can be used as feedstocks for producing renewable bio-crude oils and value-added chemicals. We elucidated the reaction pathways of a model peptide, tetra-alanine, in hot, compressed liquid water and examined the effects of pH, temperature, and time. We developed a chemical kinetic model that incorporated pH effects and estimated rate parameters from the experimental data. pH influenced the dissociation states of tetra-alanine and the selectivity of reactions. Zwitterionic tetra-alanine predominately formed di-alanine and alanine anhydride as primary products. Anionic tetra-alanine preferentially underwent hydrolysis into tri-alanine, di-alanine, and alanine. The kinetic model provided an excellent correlation to the experimental data. Highly alkaline conditions mitigated yields of alanine anhydride, a N- and O-containing heterocycle representative of compounds that undesirably partition into bio-crude oils. Accordingly, highly alkaline conditions may offer processing conditions for lessening the heteroatom content of bio-crude oils.


 Please wait while we load your content...
Please wait while we load your content...