A membrane-based electrochemical flow reactor for generation of ferrates at near neutral pH conditions†
Abstract
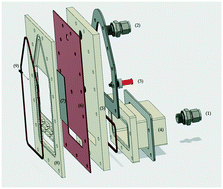
We report the electrosynthesis of Fe(VI) in a flow reactor operating in batch recirculation mode at neutral conditions using boron doped diamond (BDD) and Fe(III). The impact of several variables including current density (5–15 mA cm−2), pH (5–9), temperature (15–30 °C), and the concentration of the dissolved iron salts (3–30 mM) on the production of ferrates in the reactor were examined. In addition, the impact of a membrane in the reactor was evaluated, showing an increase on Fe(VI) generation rate and current efficiency. The rate constants for ferrate generation are affected by the initial concentration of Fe(III) and current density, and to a lesser extent by the temperature. Results show that the use of this type of reactor leads to higher current efficiency in comparison with a batch reactor, exceeding 90% for the first 25 minutes using 30 mM of Fe(III) and 10 mA cm−2. The recirculating reactor results were successfully interpreted by a simple model which considered first-order kinetics for Fe(VI) degradation.


 Please wait while we load your content...
Please wait while we load your content...