Efficient liquid-phase hydrogenolysis of a lignin model compound (benzyl phenyl ether) using a Ni/carbon catalyst†
Abstract
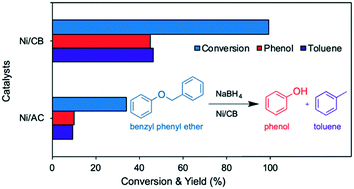
We propose an efficient liquid-phase hydrogenolysis system for the selective cleavage of an α-O-4 lignin model compound, benzyl phenyl ether (BPE), under mild conditions. By employing EtOH/H2O as co-solvent and a carbon black supported nickel (Ni/CB) catalyst in the presence of NaBH4 as the hydrogen source, BPE hydrogenolysis exhibited over 99% BPE conversion along with very high toluene (46%) and phenol (45%) yields. The catalytic performance of the Ni/CB catalyst was compared with those of various noble metal catalysts (Pd/C, Ru/C and Rh/C) for BPE hydrogenolysis. The results disclosed that the Ni/CB catalyst performs better than noble metal catalysts and exhibited higher selectivity for toluene and phenol under mild reaction conditions (80 °C). In the Ni/CB catalyst, the CB support assists in obtaining a higher yield of toluene and phenol and offers complete conversion of BPE compared to an activated carbon support (34% BPE conversion) in Ni/AC. The Ni/CB catalyst was synthesized by an impregnation method and fully characterized using various characterization techniques (XRD, SEM, TEM, XPS, ICP and FT-IR).


 Please wait while we load your content...
Please wait while we load your content...