On the prevailing reaction pathways during magnesium production via carbothermic reduction of magnesium oxide under low pressures†
Abstract
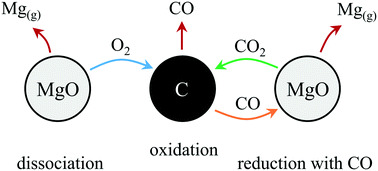
In this work we identify the prevailing reaction pathways of carbothermic reduction of MgO for the temperature and pressure ranges of 1375–1450 °C and 1–2 kPa, respectively, and normalized reduction extents of up to 0.4. It has been previously suggested that Mg(g) is produced by either (i) MgO dissociation forming O2 as the reaction intermediate or (ii) MgO(s)–C(s) boundary reaction producing CO that then reduces MgO while forming CO2 as the reaction intermediate. Either of the intermediates (O2 or CO2) are then consumed by C, which is necessary to sustain further Mg(g) production. To identify the prevailing pathways, O2 or CO2 was co-fed with Ar to sweep reacting MgO–C blends with the intent to shift the equilibrium of one of the suspected Mg(g)-producing reactions. After accounting for envisaged effects of both the C/MgO ratio in the reacting blends and the CO concentration in the reaction atmosphere, it is demonstrated that Mg(g) is produced via (1) MgO thermal dissociation and (2) MgO reduction with CO that take place in parallel. At 1375 °C and 1400 °C, roughly twice as much Mg(g) was produced via pathway (1) as compared to pathway (2). There is no evidence supporting the relevance of a direct MgO(s)–C(s) boundary reaction.


 Please wait while we load your content...
Please wait while we load your content...