Kinetic evaluation of the hydroformylation of the post-metathesis product 7-tetradecene using a bulky phosphite-modified rhodium catalyst
Abstract
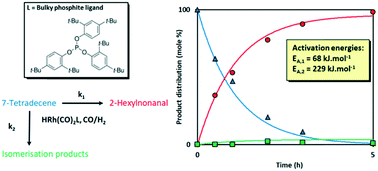
The reaction engineering kinetics for the hydroformylation of the post-metathesis product 7-tetradecene using the bulky phosphite-modified rhodium catalyst Rh-tris(2,4-di-tertbutylphenyl)phosphite were evaluated. The reaction was performed at different temperatures (60 to 90 °C), catalyst concentrations (0.5 to 1 mM), hydrogen partial pressures (15 to 25 bar) and carbon monoxide partial pressures (15 to 25 bar). The reaction system is well described by three interdependent mole balance equations in combination with a phenomenological mechanism-based rate law equation derived for the bulky phosphite ligand coordinated to rhodium. The kinetics were thus found to be first-order in alkene and catalyst concentrations (above a critical concentration), negative-order in carbon monoxide and zero-order in hydrogen. The activation energy for the hydroformylation reaction was calculated to be 68 kJ mol−1.


 Please wait while we load your content...
Please wait while we load your content...