Immobilized tetrakis(triphenylphosphine)palladium(0) for Suzuki–Miyaura coupling reactions under flow conditions†
Abstract
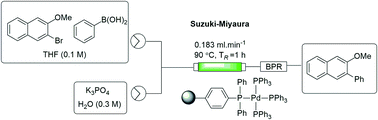
An immobilized triphenylphosphine scaffold was prepared by precipitation polymerization and functionalized to afford a cost-effective source of solid-supported tetrakis(triphenylphosphine)palladium(0). The catalyst was characterised and used to perform biphasic Suzuki–Miyaura cross-coupling reactions using a packed-bed reactor under flow conditions. The approach afforded comparable yields to those obtained under batch conditions with a single pass through the packed-bed reactor (1 h vs. 18 h). The use of a recycling system was investigated on a model reaction and found to afford close to quantitative conversion within 3 hours.
- This article is part of the themed collection: Reaction Chemistry & Engineering Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...