Unraveling the interactions in fast co-pyrolysis of microalgae model compounds via pyrolysis-GC/MS and pyrolysis-FTIR techniques†
Abstract
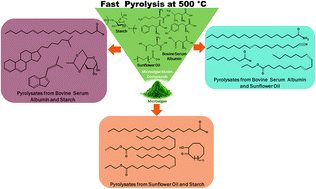
A fundamental understanding of reaction chemistry and pathways in fast pyrolysis of microalgae is hindered by the complex structure of proteins, lipids and carbohydrates that constitute them and the interactions among the intermediates at short timescales. In this study, bovine serum albumin (BSA), sunflower oil (SO) and potato starch (PS) were chosen as microalgae model compounds representing proteins, lipids, and carbohydrates, respectively. Fast pyrolysis of individual and binary mixtures of the model compounds was investigated at 500 °C using an analytical pyrolysis reactor interfaced with a gas chromatograph/mass spectrometer (GC/MS) and a Fourier transform infrared spectrometer (FTIR) to investigate the composition of pyrolysates and their time evolution. The composition of BSA, SO and PS was chosen to be 1 : 2, 1 : 1 and 2 : 1 (wt. basis) to emulate the microalgae composition. Fast pyrolysis of BSA : SO mixtures promoted esterification of carboxylic acids and alcohols, whereas BSA : PS and SO : PS mixtures promoted the formation of carboxylic acids via syn-elimination of esters, while inhibiting the decarboxylation pathway. The presence of SO and PS altered the pyrolysis mechanism of BSA by inhibiting the formation of aromatic hydrocarbons and nitrogen-containing compounds. The time evolution of C–H (aromatic and aliphatic), N–H, O–H, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations was monitored up to 60 s using in situ FTIR. The maximum vapor evolution time for fast pyrolysis of SO (50 s) was higher than that for BSA and PS (both 8–10 s). The addition of BSA and PS to SO increased the rate of evolution of volatiles, with the maximum vapor evolution occurring at shorter time periods. The first order apparent rate constants of fast pyrolysis followed the trend: 0.294 s−1 (PS) > 0.162 s−1 (BSA) > 0.107 s−1 (BSA : PS (2 : 1)) > 0.073 s−1 (BSA : SO (2 : 1)) > 0.048 s−1 (SO : PS (2 : 1)). Fast pyrolysis char was characterized by FTIR and GC/MS, and it contained polycyclic nitrogen compounds. The plausible reactions including the interactions among the various intermediates were unraveled, and a tentative mechanism was proposed.
O stretching vibrations was monitored up to 60 s using in situ FTIR. The maximum vapor evolution time for fast pyrolysis of SO (50 s) was higher than that for BSA and PS (both 8–10 s). The addition of BSA and PS to SO increased the rate of evolution of volatiles, with the maximum vapor evolution occurring at shorter time periods. The first order apparent rate constants of fast pyrolysis followed the trend: 0.294 s−1 (PS) > 0.162 s−1 (BSA) > 0.107 s−1 (BSA : PS (2 : 1)) > 0.073 s−1 (BSA : SO (2 : 1)) > 0.048 s−1 (SO : PS (2 : 1)). Fast pyrolysis char was characterized by FTIR and GC/MS, and it contained polycyclic nitrogen compounds. The plausible reactions including the interactions among the various intermediates were unraveled, and a tentative mechanism was proposed.
- This article is part of the themed collection: Reaction Chemistry & Engineering Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...