The critical role played by water in controlling Pd catalyst speciation in arylcyanation reactions†
Abstract
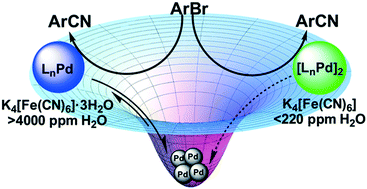
Pd-Catalyzed arylcyanation reactions are widely applied in academic and industrial problems. We demonstrate for the first time that water acts as a powerful trigger for heterogeneous catalysis in arylcyanation reactions. A selection of trans-Pd(OAc)2(HNR2)2 precatalysts have been synthesized, characterized and kinetically scrutinized as precatalysts for the Pd-catalyzed arylcyanation of aryl bromides. Kinetic and mechanistic studies using in operando infrared spectroscopic analysis reveal the key role played by water, specifically deriving from K4[Fe(CN)6]·3H2O, in facilitating a remarkable switch in mechanism from a homogeneous to heterogeneous catalytic manifold. Evidence for heterogeneity includes sigmoidal kinetic traces, an order in Pd of 0.26 and catalyst turnover sequestration upon addition of excess Hg. The TOF and TON values recorded using K4[Fe(CN)6] (<220 ppm H2O) were higher than when using K4[Fe(CN)6]·3H2O (>4000 ppm H2O). Catalytic cycles are configured according to the experimental evidence gained from this study. We conclude that water plays a critical role in Pd catalyst speciation in arylcyanation chemistry.


 Please wait while we load your content...
Please wait while we load your content...