Determination of styrene hydrogenation surface kinetics through detailed simulation of the hydrogen uptake curve†
Abstract
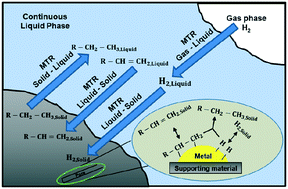
The styrene hydrogenation over Pd/C in a three-phase dead-end stirred tank reactor has been simulated. The mass transfer coefficients were calculated based on experimental data. The fast intrinsic reaction kinetics did not allow the effects of the gas–liquid and liquid–solid mass transfer to be ignored. A rigorous model is described which includes all mass transfer steps with a Langmuir–Hinshelwood model of the surface chemical reaction. The adsorption constants of hydrogen, styrene and ethylbenzene on catalyst active sites were estimated from a single experimental reaction profile. The parameterised model was validated against 6 further sets of experimental data which were not included in the parameters' estimation procedure. Results indicate the ethylbenzene, styrene and hydrogen adsorption to have an equilibrium constant of 148.34 L mol−1, 847.72 L mol−1 and 19 984 L mol−1, respectively. The intrinsic rate constant for the 4.63% Pd/C catalyst is 0.0542 mol gcat−1 s or 1.17 mol gPd−1 s−1. This work demonstrates that the analysis of the whole hydrogenation reaction profile in combination with detailed mass transfer resistance evaluation can provide fundamental system properties.


 Please wait while we load your content...
Please wait while we load your content...