A two-step telescoped continuous flow switchable process leading to nitriles, diaziridine or hydrazine derivatives†
Abstract
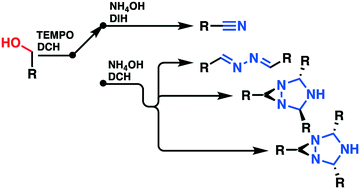
Primary and benzylic alcohols were cost-effectively transformed into their corresponding nitriles using a classical batch approach and a continuous flow process implemented on a multi-jet oscillating disk (MJOD) reactor platform. The alcohols as substrates were treated with a (2,2,6,6-tetra-methylpiperidin-1-yl)oxidanyl (TEMPO) free radical as the pre-catalyst with 1,3-dichloro-5,5-dimethylhydantoin (DCH) as the terminal oxidant to produce their corresponding carbonyl compounds. The reaction was conducted at a reaction temperature of 35 °C and a flow reactor residence time of 5 min. This alcohol to carbonyl oxidation step was telescoped with a subsequent step that involved treatment with aqueous ammonium hydroxide and 1,3-diiodo-5,5-dimethylhydantoin (DIH) at a reaction temperature of 65 °C with concomitant oxidation to nitrile using a flow reactor residence time of 15 min. A solvent exchange process was conducted in-between the two synthetic reaction steps by means of an in-line extraction process with ethyl acetate, a step that was concatenated by using an in-line liquid–liquid separation process using a hold-up tank. The second synthetic step was revealed to be tuneable, since four distinct products might be produced at varying degrees of selectivity. When DIH was used as the terminal oxidant, a high selectivity towards the original target nitrile was achieved, but if DIH was replaced with DCH as the terminal oxidant, 1,2-di((E)-benzylidene)hydrazine and two different stereoisomers of the 1,3,5-triazabicyclo[3.1.0]hexane scaffold were produced. The selectivity towards the various products was highly influenced by the reaction temperature. A scope and limitation study of the nitrile process with an assortment of primary alcohols as substrates provided excellent yields which revealed good functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...