Fluorination of neodymium carbonate monohydrate with anhydrous hydrogen fluoride in a Carberry spinning-basket reactor
Abstract
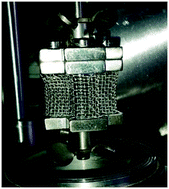
Neodymium trifluoride is used in the production of neodymium–iron–boron magnets and can be produced by the direct fluorination of neodymium carbonate monohydrate, Nd2(CO3)3·H2O, using anhydrous hydrogen fluoride, AHF. This reaction was studied in a Carberry spinning-basket reactor in order to minimise the effects of external mass transfer on the reaction kinetics, in the temperature range 100 °C to 250 °C, and at AHF concentrations up to 10 mass%. Since the monohydrate undergoes thermal decomposition, simultaneous competing reactions may take place during fluorination. There is strong evidence for the formation of partially fluorinated intermediate products. The kinetics adheres to a reaction-rate controlled, shrinking-core, heterogeneous reaction model, applicable to the individual particles comprising the pressed Nd2(CO3)3·H2O pellets used for the work. The rate is independent of temperature, and directly proportional to the AHF concentration. At 10 mass% AHF, the reaction time is 26 ± 3 min. The low operational temperature has significant techno-economic implications, lowering operating cost and costs associated with materials of construction.


 Please wait while we load your content...
Please wait while we load your content...