Non-natural 2H-azirine-2-carboxylic acids: an expedient synthesis and antimicrobial activity†
Abstract
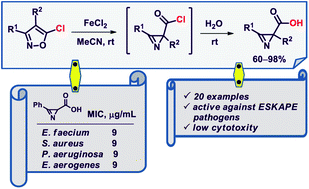
Non-natural 2H-azirine-2-carboxylic acids were obtained in high yields by FeCl2-catalyzed isomerization of 5-chloroisoxazoles to azirine-2-carbonyl chlorides followed by their hydrolysis. The 3-aryl- and 3-heteroaryl-substituted acids are stable during prolonged storage, exhibit antibacterial activity against ESKAPE pathogens and show a low level of cytotoxicity.


 Please wait while we load your content...
Please wait while we load your content...