Novel entry to the synthesis of (S)- and (R)-5-methoxycarbonylhydroxymethyluridines – a diastereomeric pair of wobble tRNA nucleosides†
Abstract
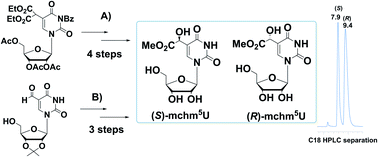
Two novel methods for the preparation of the virtually equimolar mixtures of (S)- and (R)-diastereomers of 5-methoxycarbonylhydroxymethyluridine (mchm5U) have been developed. The first method involved α-hydroxylation of a 5-malonate ester derivative of uridine (5) with SeO2, followed by transformation to (S)- and (R)-5-carboxymethyluridines (cm5U, 8) and, finally, into the corresponding methyl esters. In the second approach, (S)- and (R)-mchm5-uridines were obtained starting from 5-formyluridine derivative (9) by hydrolysis of the imidate salt (11) prepared in the acid catalyzed reaction of 5-cyanohydrin-containing uridine (10b) with methyl alcohol. In both methods, the (S)- and (R) diastereomers of mchm5U were effectively separated by preparative C18 RP HPLC.


 Please wait while we load your content...
Please wait while we load your content...