Synthesis of MeBmt and related derivatives via syn-selective ATH-DKR†
Abstract
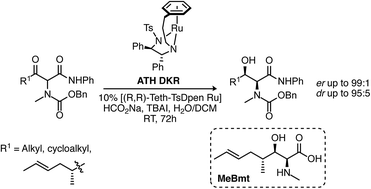
The unusual α-amino, β-hydroxy acid MeBmt is a key structural feature of cyclosporin A, an important naturally occurring immunosuppressant and antiviral agent. We present a convergent synthesis of MeBmt which relies on new aspects of dynamic kinetic resolution (DKR) to establish simultaneously the chirality at C(2) and C(3). We also show that this route is applicable to the synthesis of other derivatives.


 Please wait while we load your content...
Please wait while we load your content...