Interaction and thermal studies on graphene oxide in NC/DEGDN/GO nanocomposites
Abstract
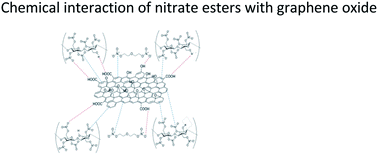
Before considering the uses of graphene oxide (GO) in nitrate ester-based materials for performance and safety improvement, its interaction, compatibility and dispersion with the host matrices need to be well understood. This work addresses the interaction and dispersity of GO with nitrocellulose (NC)/diethylene glycol dinitrate (DEGDN)-based nanocomposites. The GO and DEGDN were successfully synthesised and characterised. The NC/DEGDN proved to be a good hosting matrix for the dispersion of GO nanosheets. Analysis of atomic force microscopy (AFM) showed that the thicknesses of dispersed GO were in the range of 1–4 nm suggesting that the GO in the nanocomposite consists of 1–2 layers for a 0.5% w/w GO containing nanocomposite and 2–4 layers for a 0.75% w/w nanocomposite. ATR-FTIR spectroscopy analysis established red-shifting of 744 to 752 cm−1 for the O–NO2 bond stretching vibrations, indicating bond stabilization by donor electron from the GO. The Raman spectra analysis showed GO peaks blue-shifting and broadening which is attributed to hydrogen bonding interaction between GO sheets and –NO2 groups. The activation energy of nitrate ester decomposition of NC/DEGDN/GO nanocomposites increases as a function of GO content from 167 kJ mol−1 and reaches a maximum of 214 kJ mol−1 for a 0.5% w/w GO loading. This suggests an improvement of the nitrate ester bond stability. These findings open a new direction to the application of GO in nitrate ester-based materials for increased stability, safety and shelf life.


 Please wait while we load your content...
Please wait while we load your content...