Theoretical investigation of various aspects of two dimensional holey boroxine, B3O3†
Abstract
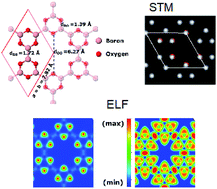
By means of first-principles calculations, we study the structural, electronic and mechanical properties of the newly synthesized boron–oxygen holey framework (Chem. Comm. 2018, 54, 3971). It has a planar structure formed by B3O3 hexagons, which are joined via strong covalent boron–boron bonds. The six B3O3 units are connected with six-fold symmetry exhibiting a large hole with a surface area of 23 Å2, which is ideal for the adsorption of alkalis. For neutral alkalis, we found that the adsorption energy of potassium is 14 and 12 kcal mol−1 larger than those determined for sodium and lithium, respectively. In contrast, for alkali cations, there is a clear preference for lithium over sodium and potassium. With regard to its electronic properties, it is an insulator with an electronic band gap of 5.3 eV, at the HSE level of theory. We further investigate the effect of strain on the band gap and find it a less efficient technique to tune the electronic properties. The wide optical gap of B3O3 can be utilized in ultraviolet (UV) applications, such as UV photodetectors, etc. Additionally, the 2D elastic modulus of B3O3 (53.9 N m−1) is larger than that of Be3N2, silicene, and germanene. Besides, we also report bilayer and graphite-like bulk B3O3 and furthermore, find that the optoelectronic properties of the bilayer can be tuned with an external electric field. The great tunability of optical properties from UV to the visible range offers a vast range of applications in optoelectronics.


 Please wait while we load your content...
Please wait while we load your content...