Experimental and theoretical insights into the corrosion inhibition activity of novel Schiff bases for aluminum alloy in acidic medium†
Abstract
Three novel Schiff bases, namely N-(4-((4-((phenylimino)methyl)phenoxy)methoxy)benzylidene)benzenamine (UA), N-(3-methoxy-4-((2-methoxy-4-((phenylimino)methyl)phenoxy)methoxy)benzylidene)benzenamine (UB), and N-(3-ethyl-4-((2-ethyl-4-((phenylimino)methyl)phenoxy)methoxy)benzylidene)benzenamine (UC), were synthesized and their structures were elucidated through diverse spectroscopic techniques such as FT-IR, GC-MS, 1H NMR and 13C NMR. The corrosion inhibition effect of these Schiff bases on aluminum alloy AA2219-T6 in acidic medium was explored using weight loss, Tafel polarization, and electrochemical impedance spectroscopy. Theoretical quantum chemical calculations using density functional theory were employed to determine the adsorption site. It was found that inhibition efficiencies increase with an increase in the inhibitor concentration. Tafel plots showed that these Schiff bases function as mixed inhibitors. Adsorption of the Schiff bases on aluminum followed the Langmuir adsorption isotherm and the value of 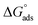 showed a dominant chemical mechanism. FT-IR and SEM techniques were used to investigate the surface morphology. The compounds showed a substantial corrosion inhibition for aluminum alloy in 0.1 M HCl at 298 K. UB and UC exhibited superior anticorrosion efficiency compared to UA originating from the electron-donating methoxy and ethoxy group substitutions, respectively. There was found to be good correlation between molecular structure and inhibition efficiencies.
showed a dominant chemical mechanism. FT-IR and SEM techniques were used to investigate the surface morphology. The compounds showed a substantial corrosion inhibition for aluminum alloy in 0.1 M HCl at 298 K. UB and UC exhibited superior anticorrosion efficiency compared to UA originating from the electron-donating methoxy and ethoxy group substitutions, respectively. There was found to be good correlation between molecular structure and inhibition efficiencies.



 Please wait while we load your content...
Please wait while we load your content...