UV light promoted ‘Metal’/‘Additive’-free oxidation of alcohols: investigating the role of alcohols as electron donors†
Abstract
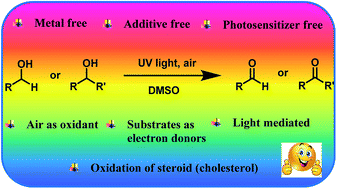
UV light promoted selective oxidation of primary and secondary alcohols has been demonstrated under ‘metal-free’ and ‘additive-free’ conditions. Under the optimized conditions, a variety of aromatic, heteroaromatic, and alicyclic alcohols have been examined for their transformations to the corresponding carbonyl compounds. The mechanistic studies emphasize the important role of substrate (alcohol) and solvent (DMSO) in the generation of superoxide radical which is a vital intermediate for the transformation. This study also highlights the role of air as the oxidant in the oxidation process. Further, the practical application of the strategy has also been demonstrated for the oxidation of the alcoholic moiety in cholesterol.
- This article is part of the themed collection: How does it work? – a collection on mechanistic studies


 Please wait while we load your content...
Please wait while we load your content...