Dissolution of metal oxides in task-specific ionic liquid†
Abstract
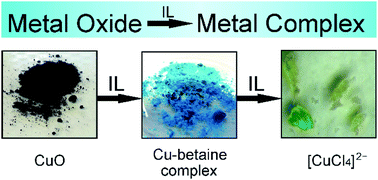
Due to their typically low reactivity, the activation of metal oxides, as found in ores, earths and minerals, is in general performed by high temperature reactions, which consume much energy. Owing to the prevalence of fossil fuels, this is accompagnied by the generation of large amounts of CO2. Alternatively, ionic liquids (ILs) can be used as solvents for metal oxide dissolution and downstream chemistry at much lower temperatures. The dissolution ability of the dry ionic liquid betainium bis(trifluoromethylsulfonyl)imide, [Hbet][NTf2], was investigated for 30 metal oxides at 175 °C and compared to chloride containing IL [Hbet]2[NTf2]Cl. A general dissolution-promoting effect of chloride anions was found, regarding reaction time as well as the variety of dissolved metal oxides. Up to now, the dissolution in [Hbet]2[NTf2]Cl is limited to basic or amphoteric metal oxides and assumed to be influenced by multiple factors, such as reaction conditions and the lattice energy of the metal oxide as well as its crystal structure. Comprehensive investigations were performed for the dissolution of CuO, which led to the discovery of the water-free complex compound [Cu2(bet)4(NTf2)2][NTf2]2. Proceeding from this compound, a complete exchange of the O-coordination sphere by other ligands was demonstrated, opening up promising possibilities for downstream chemistry.


 Please wait while we load your content...
Please wait while we load your content...