Triphala inhibits alpha-synuclein fibrillization and their interaction study by NMR provides insights into the self-association of the protein†
Abstract
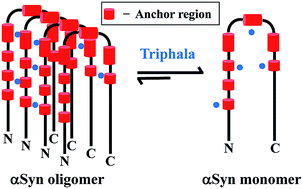
The process of assembly and accumulation of the intrinsically disordered protein (IDP), alpha-synuclein (αSyn) into amyloid fibrils is a pathogenic process leading to several neurodegenerative disorders such as Parkinson's disease, multiple system atrophy and others. Although several molecules are known to inhibit αSyn fibrillization, the mechanism of inhibition is just beginning to emerge. Here, we report the inhibition of fibrillization of αSyn by Triphala, a herbal preparation in the traditional Indian medical system of Ayurveda. Triphala was found to be a rich source of polyphenols which are known to act as amyloid inhibitors. ThT fluorescence and TEM studies showed that Triphala inhibited the fibrillization of αSyn. However, it was observed that Triphala does not disaggregate preformed αSyn fibrils. Further, native-PAGE showed that Triphala reduces the propensity of αSyn to oligomerize during the lag phase of fibrillization. Our NMR results showed that certain stretches of residues in the N-terminal and NAC regions of αSyn play an anchor role in the self-association process of the protein, thereby providing mechanistic insights into the early events during αSyn fibrillization.


 Please wait while we load your content...
Please wait while we load your content...