In situ mass change and gas analysis of 3D manganese oxide/graphene aerogel for supercapacitors†
Abstract
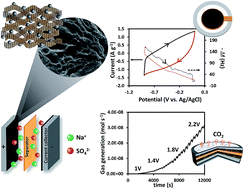
Manganese oxide nanoparticles decorated on 3D reduced graphene oxide aerogels (3D MnOx/rGOae) for neutral electrochemical capacitors were successfully produced by a rapid microwave reduction process within 20 s. The symmetric electrochemical capacitor of 3D MnOx/rGOae (Mn 3.0 at%) storing charges via both electric double layer capacitance (EDLC) and pseudocapacitance mechanisms exhibits a specific capacitance of 240 F g−1 as compared with 190 F g−1 of that using the bare 3D rGOae at 0.5 A g−1 in 1 M Na2SO4 (aq.) electrolyte. It retains 90% of the initial capacitance after 10 000 cycles, demonstrating high cycling stability. In addition, the charge storage mechanism of 3D MnOx/rGOae was investigated using an electrochemical quartz crystal microbalance. In situ gas analysis using differential electrochemical mass spectrometry (DEMS) shows the CO2 evolution at a cell potential over 1 V indicating that the positive electrode is possibly the voltage limiting electrode in the full cell. This finding may be useful for further development of practical high power and energy supercapacitors.


 Please wait while we load your content...
Please wait while we load your content...