Synthesis of 1,4,5,6-tetrahydropyridazines and pyridazines via transition-metal-free (4 + 2) cycloaddition of alkoxyallenes with 1,2-diaza-1,3-dienes†
Abstract
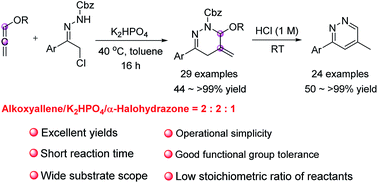
We developed an economical and practical protocol for the synthesis of 1,4,5,6-tetrahydropyridazines. A diverse range of alkoxyallenes and 1,2-diaza-1,3-dienes undergo (4 + 2) cycloaddition to generate the desired products in excellent yields. The high efficiency, wide substrate scope and good functional group tolerance of this process, coupled with operational simplicity, render the method synthetically attractive. The utility of the cycloaddition is also demonstrated by the preparation of various pyridazines from 1,4,5,6-tetrahydropyridazines.


 Please wait while we load your content...
Please wait while we load your content...