Structure and dynamics of γ-secretase with presenilin 2 compared to presenilin 1†
Abstract
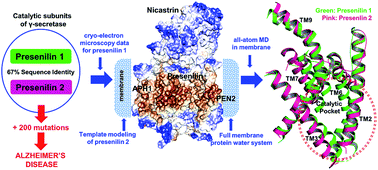
Severe early-onset familial Alzheimer's disease (FAD) is caused by more than 200 different mutations in the genes coding for presenilin, the catalytic subunit of the 4-subunit protease complex γ-secretase, which cleaves the C99 fragment of the amyloid precursor protein (APP) to produce Aβ peptides. γ-Secretase exists with either of two homologues, PS1 and PS2. All cryo-electron microscopic structures and computational work has so far focused on γ-secretase with PS1, yet PS2 mutations also cause FAD. A central question is thus whether there are structural and dynamic differences between PS1 and PS2. To address this question, we use the cryo-electron microscopic data for PS1 to develop the first structural and dynamic model of PS2-γ-secretase in the catalytically relevant mature membrane-bound state at ambient temperature, equilibrated by three independent 500 ns molecular dynamics simulations. We find that the characteristic nicastrin extra-cellular domain breathing mode and major movements in the cytosolic loop between TM6 and TM7 occur in both PS2- and PS1-γ-secretase. The overall structures and conformational states are similar, suggesting similar catalytic activities. However, at the sequence level, charge-controlled membrane-anchoring is extracellular for PS1 and intracellular for PS2, which suggests different subcellular locations. The tilt angles of the TM2, TM6, TM7 and TM9 helices differ in the two forms of γ-secretase, suggesting that the two proteins have somewhat different substrate processing and channel sizes. Our MD simulations consistently indicated that PS2 retains several water molecules near the catalytic site at the bilayer, as required for catalysis. The possible reasons for the differences of PS1 and PS2 are discussed in relation to their location and function.


 Please wait while we load your content...
Please wait while we load your content...