Gram-scale carbasugar synthesis via intramolecular seleno-Michael/aldol reaction†
Abstract
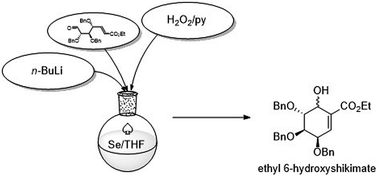
Carbasugars represent an important category of natural products possessing a broad spectrum of biological activities. Lots of effort has been done to develop gram scale synthesis. We are presenting a new approach to gram scale synthesis of the carbasugar skeleton via intramolecular seleno-Michael/aldol reaction. The proposed strategy gave gram amounts of 6-hydroxy shikimic ester in a tandem process in 36% overall yield starting from D-lyxose. We have attempted to demonstrate the synthetic utility of 6-hydroxyshikimic acid derivatives by covering the important synthetic modifications and related applications, namely synthesis of protected (−)-gabosine E, (−)-MK7606, (−)-valienamine and finally unprotected methyl (−)-shikimate.


 Please wait while we load your content...
Please wait while we load your content...