Mechanistic insight into the catalytic hydrogenation of nonactivated aldehydes with a Hantzsch ester in the presence of a series of organoboranes: NMR and DFT studies†
Abstract
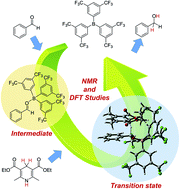
Mechanistic studies on the organoborane-catalyzed transfer hydrogenation of nonactivated aldehydes with a Hantzsch ester (diethyl-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate) as a synthetic NADH analogue were performed by NMR experiments and DFT calculations. In the reaction of benzaldehyde with the Hantzsch ester, the catalytic activity of tris[3,5-bis(trifluoromethyl)phenyl]borane was superior to that of other borane catalysts, such as tris(pentafluorophenyl)borane, trifluoroborane etherate, or triphenylborane. Stoichiometric NMR experiments demonstrated that the hydrogenation process proceeds through activation of the aldehyde by the borane catalyst, followed by hydride transfer from the Hantzsch ester to the resulting activated aldehyde. DFT calculations for the hydrogenation of benzaldehyde with the Hantzsch ester in the presence of borane catalysts supported the reaction pathway and showed why the catalytic activity of tris[3,5-bis(trifluoromethyl)phenyl]borane is higher than that of the other boron catalysts. Association constants and Gibbs free energies in the reaction of boron catalysts with benzaldehyde or benzyl alcohol, which were investigated by 1H NMR analyses, also indicated why tris[3,5-bis(trifluoromethyl)phenyl]borane is a superior catalyst to tris(pentafluorophenyl)borane, trifluoroborane etherate, or triphenylborane in the hydrogenation reaction.


 Please wait while we load your content...
Please wait while we load your content...