CF3: an overlooked chromophore in VCD spectra. A review of recent applications in structural determination†‡
Abstract
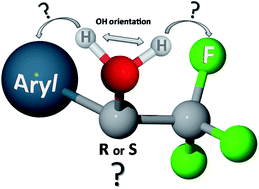
The VCD spectra of several chiral compounds containing the CF3 group are reviewed and analyzed. The list of compounds contains pharmaceutically relevant molecules as well as simple model molecules, having the value of case studies. In particular we point out the importance of the sign of the VCD band relative to some stretching normal mode of CF3 in the region 1110–1150 cm−1, as diagnostic of the configuration of stereogenic carbons C* to which the CF3 group is bound: the correspondence (−) ↔ (R) and (+) ↔ (S) holds for 100% of 1-aryl-2,2,2-trifluoroethanols. DFT calculations confirm these conclusions, but for the rule established here they serve just as a check. This rule is tested on two new compounds, namely N-tert-butanesulfinyl-1-(quinoline-4-yl)-2,2,2-trifluoroethylamine, 8, and 4-[2-(2,2,2-trifluoro-1-hydroxyethyl)pyrrolidin-1-yl]-2-(trifluoromethyl)benzonitrile, 10, both containing two stereogenic elements, one of them being an asymmetric carbon C* of unknown configuration binding a CF3 group. Discussion of the general validity of the rule is provided and some further tests are run on compounds in well-established drugs.


 Please wait while we load your content...
Please wait while we load your content...