Hydrophobics of CnTAB in an aqueous DMSO–BSA nanoemulsion for the monodispersion of flavonoids†
Abstract
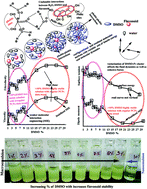
Herein, philicphobic interactions between flavonoids (quercetin, apigenin, and naringenin) and bovine serum albumin (BSA) were analyzed using physicochemical properties obtained at T = 298.15, 303.15, 308.15 K and 0.1 MPa, from 0.01 to 0.10 mol kg−1 of alkyl trimethyl ammonium bromide (CnTAB : DTAB, Cn = 12; TDTAB, Cn = 14; HDTAB, Cn = 16). The flavonoids with cationic surfactants strongly interacted with BSA, as illustrated by the physicochemical parameters (PCPs), refractive index (nD), Walden product, pH, electrostatic potential and molar conductance (Λm). Viscosity (η), density (ρ), ηD, sound velocity (u) and specific conductance (k) data were used to calculate the relative viscosity (ηr), viscous relaxation time (τ), Walden product, entropy (ΔS), enthalpy (ΔH), Gibbs free energy (ΔG), heat capacity (Δq) limiting dielectric constant (ε∞), speed of light (C), acoustic impedance (Z) and molar refraction (R). These PCPs have quantitatively predicted the hydrophilic and hydrophobic (philicphobic) interactions developed are on increasing the alkyl chain (AC) of CnTAB. These interactions assist a monodispersion of the flavonoids, and a similar mechanism could equally be applicable to monodisperse the antioxidants in the aqueous nanoemulsions. Their philicphobic stoichiometry weakened the cohesive forces (CF) when the shear stress was increased, and enhanced surface activities were achieved that facilitated the flavonoids to interact with BSA due to intermolecular forces (IMF) to develop a stable nanoemulsion; Upon increasing the CnTAB concentrations, the nD value increases since the polarizability increases with stronger shear stress due to van der Waal forces and electrostatic interactions to achieve better flavonoid–BSA linkages.


 Please wait while we load your content...
Please wait while we load your content...