Novel mono-lipidated dimeric glucagon-like peptide-1 receptor agonist with improved long-acting and hypoglycemic activity†
Abstract
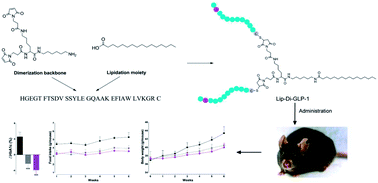
Dimerization is a useful tool to boost ligand–receptor interaction. Both lipidation and dimerization effectively prolong the short half-life (t1/2) of peptides by facilitating binding with serum albumin and increasing hydrodynamic size. Here, we described two novel GLP-1 conjugates with high glucagon-like peptide-1 (GLP-1) receptor activation potencies, dimerized GLP-1 (Di-GLP-1) and lipidated Di-GLP-1 (Lip-Di-GLP-1). Di-GLP-1 and Lip-Di-GLP-1 were prepared through cysteine–maleimide specific coupling reactions using Gly8-Cys31-GLP-1, bis-maleimide amine, and activated palmitic acid. The receptor activation potencies of Di-GLP-1 and Lip-Di-GLP-1 were 13.6-fold and 9.5-fold higher than GLP-1, respectively. The in vivo hypoglycemic and insulinotropic activities of Di-GLP-1 and Lip-Di-GLP-1 were also better than GLP-1 in db/db mice. Furthermore, Lip-Di-GLP-1 was found to have greater circulating t1/2 than synthesized liraglutide by 1.8-fold. Accordingly, the improved pharmacokinetic profiles of Lip-Di-GLP-1 resulted in protracted antidiabetic effects as confirmed by hypoglycemic duration test. Moreover, Lip-Di-GLP-1 administered in mice potently inhibits gastric emptying and reduce food intake. Chronic Lip-Di-GLP-1 treatment in db/db mice resulted in significant improvements in food intake, body weight, pancreatic function and corrected hyperglycemia, which was more effective than synthesized liraglutide. Our research indicated that combined dimerization and lipidation were effectively applied to GLP-1, and the preclinical results suggested the potential usage of Lip-Di-GLP-1 as a long-acting antidiabetic agent.


 Please wait while we load your content...
Please wait while we load your content...