A highly selective colorimetric fluorescent probe for detection of Hg2+ and its application on test strips
Abstract
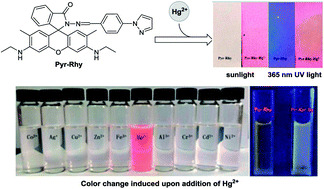
An efficient fluorescent probe Pyr-Rhy based on pyrazole was developed, which can detect Hg2+ in water. Its fluorescence properties were studied by UV-vis and fluorescence spectroscopy, and the study results indicated that this probe can selectively detect Hg2+via complexation reaction, and then cause a remarkable color change from colorless to pink and a strong fluorescence enhancement can be observed. Furthermore, this probe showed high sensitivity with the detection limit down to 2.07 × 10−8 M, and its stoichiometric ratio toward Hg2+ ions was 1 : 1. The sensing mechanism was investigated by Job's plot 1H NMR titrations, and FT-IR spectra analysis, which demonstrated a chelation-enhanced fluorescence (CHEF) mechanism. More importantly, obvious color changes of sensor Pyr-Rhy can be observed when it was impregnated on filter paper testing strips and immersed in Hg2+ solution (water as solution), indicating its potential application for trace Hg2+ detection in environmental samples.
- This article is part of the themed collection: Editors’ collection: Fluorescent Sensors


 Please wait while we load your content...
Please wait while we load your content...