Discovery of human TyrRS inhibitors by structure-based virtual screening, structural optimization, and bioassays†
Abstract
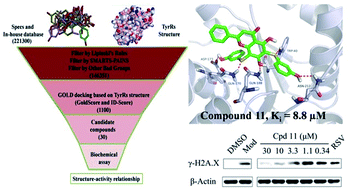
The human tyrosyl transfer-RNA (tRNA) synthetase (TyrRS), which is well known for its essential aminoacylation function in protein synthesis, has been shown to translocate to the nucleus and protect against DNA damage caused by external stimuli. Small molecules that can fit into the active site pocket of TyrRS are thought to affect the nuclear role. The exploitation of TyrRS inhibitors has attracted attention recently. In this investigation, we adopted a structure-based virtual screening strategy and subsequent structure–activity relationship analysis to discover new TyrRS inhibitors, and identified a potent compound 5,7-dihydroxy-6,8-bis((3-hydroxyphenyl)thio)-2-phenyl-4H-chromen-4-one (compound 11, Ki = 8.8 μM). In intact HeLa cells, this compound showed a protective effect against DNA damage. Compound 11 is a good lead compound for the further development of drugs against disorders caused by DNA damage.
- This article is part of the themed collection: Editors' collection: Chemical Biology


 Please wait while we load your content...
Please wait while we load your content...