K3PO4-promoted domino reactions: diastereoselective synthesis of trans-2,3-dihydrobenzofurans from salicyl N-tert-butanesulfinyl imines and sulfur ylides†‡
Abstract
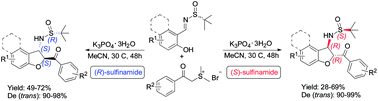
An efficient domino annulation between sulfur ylides and salicyl N-tert-butylsulfinyl imines was developed. The reaction proceeds with a diastereodivergent process, the configuration of the sulfinyl group determining the stereochemical course of the reaction. The method allows the synthesis of a highly substituted trans-2,3-dihydrobenzofuran skeleton with high yield and good chemo- and diastereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...