Novel cathepsin K inhibitors block osteoclasts in vitro and increase spinal bone density in zebrafish†
Abstract
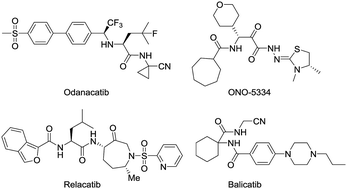
Cathepsin K (Cat K) is a predominant cysteine protease and highly potent collagenase expressed in osteoclasts. Cat K inhibitors are anti-resorptive agents to treat osteoporosis. A novel scaffold of cathepsin K inhibitors, exemplified by lead compound 1x, was used as the template for designing and synthesizing a total of 61 derivatives that have not been reported before. An exploratory structure–activity relationship analysis identified the potent Cat K inhibitor A22, which displayed an IC50 value of 0.44 μM against Cat K. A22 was very specific for Cat K and caused a significantly higher in vitro inhibition of the enzyme as compared to that of lead compound 1x. A surface plasmon resonance analysis confirmed in vitro binding of A22 to Cat K. Molecular docking studies indicated several favourable interaction sites for A22 within the active pocket of Cat K. Furthermore, A22 also blocked active osteoclasts in vitro and increased spinal bone density in zebrafish, in which it showed an activity that was higher than that of the marketed therapeutic bone metabolizer etidronate disodium. A22 represents a very promising lead compound for the development of novel antiresorptive agents functioning as orthosteric inhibitors of Cat K.
- This article is part of the themed collection: Editors' collection: Chemical Biology


 Please wait while we load your content...
Please wait while we load your content...