Design, synthesis and broad-spectrum Bcr-Abl inhibitory activity of novel thiazolamide–benzamide derivatives†
Abstract
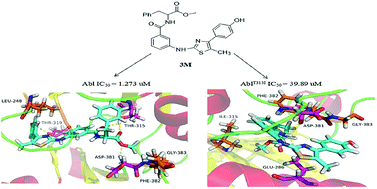
Bcr-Abl plays an important role in the pathogenesis and development of chronic myeloid leukemia (CML). But Bcr-Abl is prone to mutation, so it increases the difficulty of clinical treatment. Therefore, it is crucial to design a new class of broad-spectrum Bcr-Abl inhibitors. Herein, forty novel thiazolamide–benzamide derivatives were synthesized and evaluated their broad-spectrum Bcr-Abl inhibitory activities. The newly synthesized compounds were characterized by using spectrum data (1H NMR, APCI-MS and IR) and elemental analysis. The protein kinase results indicated that eight compounds (3a, 3e, 3m, 3n, 3p, 4c, 4f, 4g) showed high activities to wild-type and T315I mutation. The most potent compound 3m exhibited an Abl IC50 value as low as 1.273 μM and showed inhibition to the T315I mutant with IC50 value 39.89 μM. 3m could prove to be a new promising lead compound for the further development of broad-spectrum Bcr-Abl inhibitors to overcome clinical acquired resistance.
- This article is part of the themed collection: Editors' collection: Chemical Biology


 Please wait while we load your content...
Please wait while we load your content...