Aggregation-induced chiroptical generation and photoinduced switching of achiral azobenzene-alt-fluorene copolymer endowed with left- and right-handed helical polysilanes†
Abstract
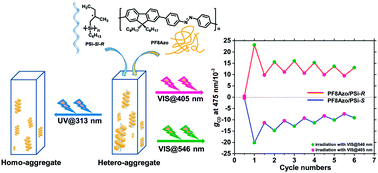
The left and right helicities of azobenzene (Azo)-containing main-chain polymer (PF8Azo) were successfully controlled with an enantiomeric pair of rigid rod-like helical polysilanes carrying (S)- and (R)-2-methylbutyl groups (PSi-S and PSi-R, respectively) as their hetero-aggregates in a mixture of chloroform and methanol solvents and in the solid state. Optimizing the good and poor cosolvents and their volume fractions showed that the molar ratio of PF8Azo to PSi-S/-R and the molecular weight of PF8Azo were crucial to boost the CD amplitudes of PF8Azo/PSi-S and PF8Azo/PSi-R hetero-aggregates. The photoresponsive trans–cis transformation caused noticeable changes in the sign and magnitude of the chiroptical behavior due to the hetero-aggregates. Moreover, the optically active PF8Azo homo-aggregates were produced by complete photoscissoring reactions at 313 nm, which could be assigned to the Siσ–Siσ* transitions of PSi-S and PSi-R.


 Please wait while we load your content...
Please wait while we load your content...