Photostability and photocatalytic degradation of ionic liquids in water under solar light†
Abstract
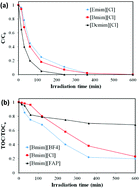
The aim of this work is to study, (i) the photostability of different imidazolium and pyridinium ionic liquids (ILs) in water under solar light; and (ii) the photocatalytic degradation of those ILs in water with TiO2 under solar light. The effects of the type of cation and anion as well as the length of the cationic chain of the imidazolium ILs have been analyzed. These imidazolium-based ILs show high solar stability, slightly decreasing as the length of the cationic chain increases. The anion plays a main role in the stability of ILs under solar light, decreasing in the case of hydrophobic anions. The kind of head group (pyridinium or imidazolium) or the presence of functional groups (allyl, OH) also influence the solar light stability. DFT calculations on the fundamental and excited electronic states of the ILs were carried out to obtain a deeper insight on their photostability. In the case of the photocatalytic degradation of the ILs, complete conversion was achieved for all the ILS tested but mineralization reached 80% at the most. The rate of degradation increased with the length of the alkyl chain while the anion showed little effect. The pyridinium-based IL tested was the easiest to breakdown.


 Please wait while we load your content...
Please wait while we load your content...