Transition-metal free direct C–H functionalization of quinoxalin-2(1H)-ones with oxamic acids leading to 3-carbamoyl quinoxalin-2(1H)-ones†
Abstract
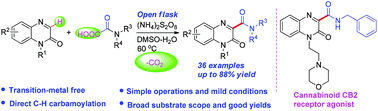
A practical transition-metal free decarboxylative coupling reaction of oxamic acids with quinoxalin-2(1H)-ones has been developed under mild conditions. This transformation provides an efficient approach for the preparation of 3-carbamoyl quinoxalin-2(1H)-ones, which are important subunits in biologically active natural products and medicinal chemistry. This protocol features broad substrate scope, good yields, and mild reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...