Lewis acid-catalyzed tandem cyclization of in situ generated o-quinone methides and arylsulfonyl hydrazides for a one-pot entry to 3-sulfonylbenzofurans†
Abstract
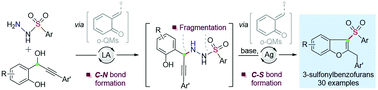
A Lewis acid-catalyzed one-pot aza-Michael addition/fragmentation/sulfinyl conjugate addition/cyclization cascade of o-QMs and arylsulfonyl hydrazides is described for the first time. This technology engages a broad range of substrates with both components under mild conditions, giving the corresponding valuable 3-sulfonylbenzofurans in generally good yields. These investigations show the use of arylsulfonyl hydrazides as nucleophiles for the first time in catalytic C–N and C–S bond formation with o-QMs.


 Please wait while we load your content...
Please wait while we load your content...