Visible-light-mediated de-aminative alkylation of N-arylamines with alkyl Katritzky salts†
Abstract
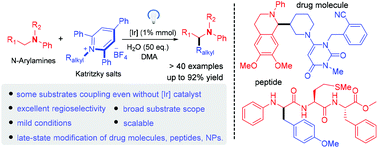
A visible-light-mediated de-aminative alkylation of N-arylamines has been developed, providing direct access to α-amino C–H functionalization from readily available Katritzky salts under mild conditions. In some cases, this operationally simple protocol can be performed in the absence of a photocatalyst. A wide range of substrates and functional groups are tolerated. The utility of this approach is highlighted by its application to the late-state modification of drug molecules, natural products and peptides.


 Please wait while we load your content...
Please wait while we load your content...