K2S2O8-promoted direct thiocyanation of pyrazolin-5-ones with ammonium thiocyanate at room temperature†
Abstract
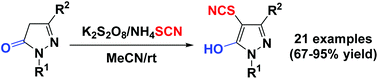
A facile and efficient approach for the direct thiocyanation of pyrazolin-5-ones via a radical mechanism has been established for the first time. A series of 4-thiocyanated 5-hydroxy-1H-pyrazoles were synthesized in fair to good yields by K2S2O8-promoted direct thiocyanation of pyrazolin-5-ones with NH4SCN at room temperature under simple and mild reaction conditions with atom- and step-economy and good functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...