(+)- and (−)-actinoxocine, and actinaphthorans A–B, C-ring expansion and cleavage angucyclinones from a marine-derived Streptomyces sp.†
Abstract
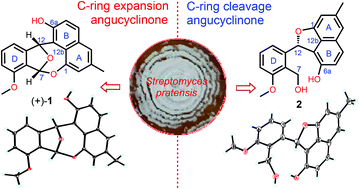
(+)- and (−)-actinoxocine (1), a pair of enantiomeric C-ring expansion angucyclinones, featuring a unique epoxybenzo[f]naphtho[1,8-bc]oxocine carbon skeleton, and two unusual C-ring cleavage analogues, actinaphthorans A–B (2–3), were isolated from a marine-derived Streptomyces sp. Their structures and absolute configurations were assigned by spectroscopic analysis, X-ray diffraction and CD calculations.


 Please wait while we load your content...
Please wait while we load your content...